CLINICAL STUDIES
The VALOR trial
The VALOR trial (Study 1 Part C) lasted 28 weeks, and the results were analyzed at the end of the study. At the end of VALOR, all remaining participants were given the opportunity to continue in the open-label extension (OLE) study. All participants enrolled in the OLE study were treated with QALSODY.
There was an initial analysis of the OLE study when all participants had the opportunity to complete 52 weeks of treatment. This allowed the researchers to compare results of participants who started QALSODY at the start of the VALOR study to those who only received QALSODY later during the OLE study.
How QALSODY was studied in the VALOR trial:
- Placebo controlled, which means the study was designed to compare QALSODY to placebo drug (an inactive substance that looks the same as, and is given the same as, an active drug being tested). Everything else in the study was the same for all participants. Then, researchers compare the effects in each group to determine whether the new treatment works. This allows researchers to understand if QALSODY had any effect on the study outcomes
- Randomized 2:1, which means that participants were randomly assigned to receive QALSODY or the placebo. Twice as many participants were given QALSODY as placebo
- Double-blind, which means neither the participants nor the study doctors or staff knew if the participants received QALSODY or the placebo
Who participated in VALOR?
VALOR studied the effectiveness and safety of QALSODY compared to placebo in people with weakness due to amyotrophic lateral sclerosis (ALS) who have a mutation, or change, in the superoxide dismutase 1 (SOD1) gene.
A total of 108 people with SOD1-ALS participated in VALOR
Participants received treatment for 24 weeks (3 loading doses followed by 5 maintenance doses).
Participants were allowed to also take riluzole and/or edaravone during the study.
VALOR participants were determined to either have faster progressing SOD1-ALS or slower progressing SOD1-ALS
The faster progressing group was the Primary analysis population that included 60 participants who met the following conditions:
- Had a slow vital capacity, or SVC, (a measure of breathing function) ≥65% of percent Predicted value
- Exhibited more rapid decline based on the ALSFRS-R before the start of the study
- Had a specific type of SOD1 mutation associated with faster disease progression
The slower progressing group included 48 participants who met the following criteria:
- Had an SVC (a measure of breathing function) ≥50% of Predicted value
- Did not meet criteria for inclusion in the Primary analysis population
What were participants’ baseline characteristics?
Baseline disease characteristics
- Baseline disease characteristics were generally similar between those treated with QALSODY and those who received placebo. However, the QALSODY group had:
- Slightly shorter time from when symptoms began
- Higher blood levels of neurofilament (a blood-based marker of nerve damage and deterioration) at the start of the study
- 62% of participants were taking riluzole, and 8% of participants were taking edaravone
- The average ALSFRS-R score at the start of the study was 36.9 in the QALSODY treatment group and 37.3 in the placebo group
- The average time from symptoms beginning was 49.5 weeks in the QALSODY treatment group and 63.4 weeks in the placebo group
What question was VALOR designed to answer?
The primary outcome, or main question VALOR was designed to answer, was:
In participants who met the criteria for faster progressing disease, how much did their ALSFRS-R scores
change at 28 weeks compared to the start of the study?
- These results were examined using statistical methods to reduce the risk of bias or unsupportable conclusions that may have been caused by patient deaths during the trial, as well as to account for missing data (for withdrawals other than death)
VALOR results
What were the results of VALOR?
experienced less decline in average ALSFRS-R
scores compared to the placebo group, but the
results were not Statistically significant (QALSODY-placebo Adjusted mean difference [95% CI]: 1.2 [-3.2, 5.5]).
Why was QALSODY approved?
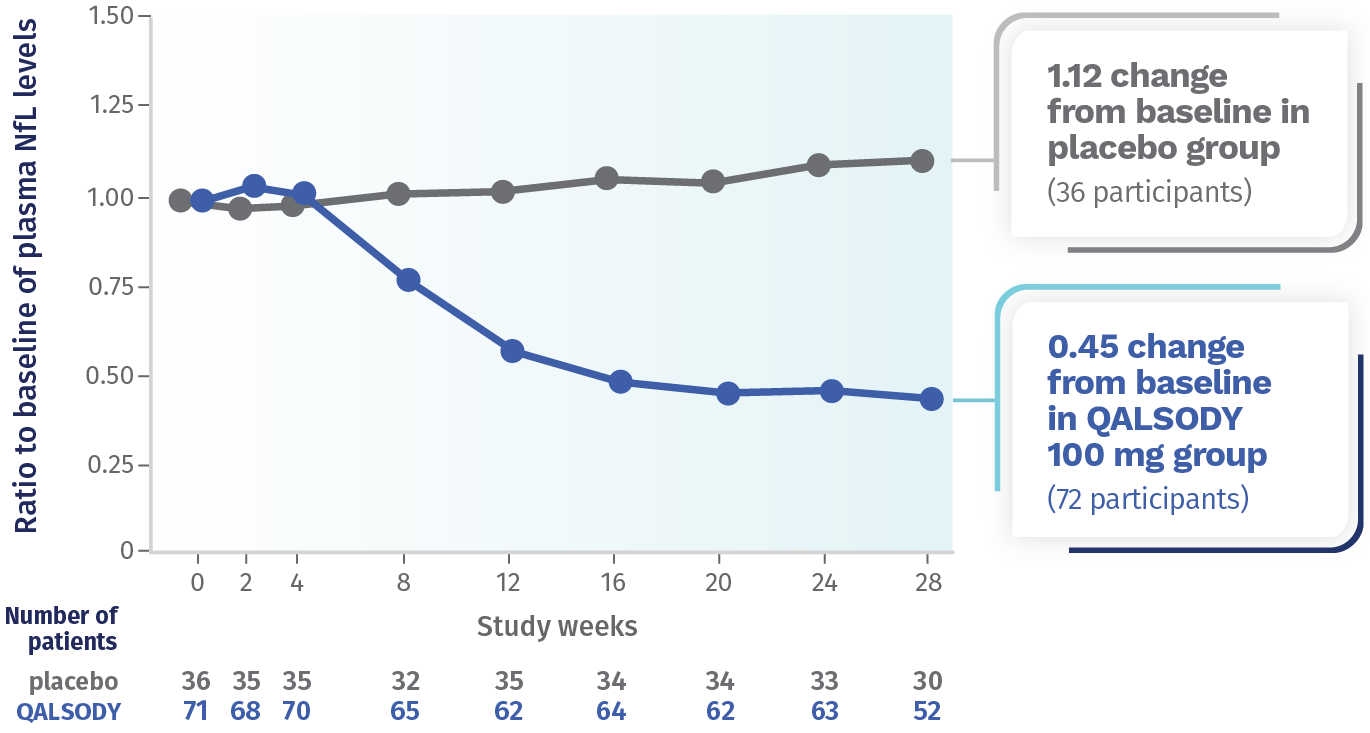
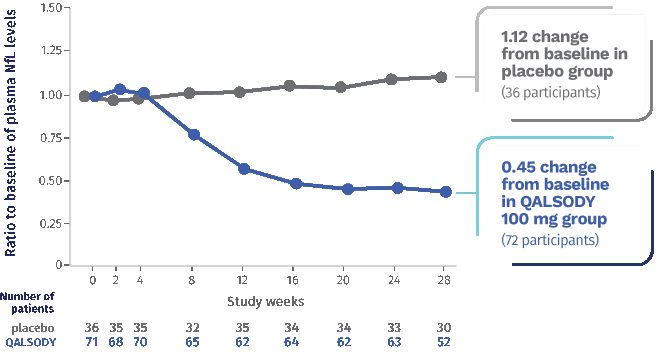
Neurofilament light chain, or NfL, is a blood-based biomarker of nerve damage and deterioration.
QALSODY® (tofersen) is a prescription medicine used to treat adults with amyotrophic lateral sclerosis (ALS) who have a mutation, or change, in the superoxide dismutase 1 (SOD1) gene. QALSODY is approved under accelerated approval based on reduction in NfL in the blood observed in patients treated with QALSODY. Continued approval for QALSODY may depend on results of additional studies to confirm that there is a clinical benefit.
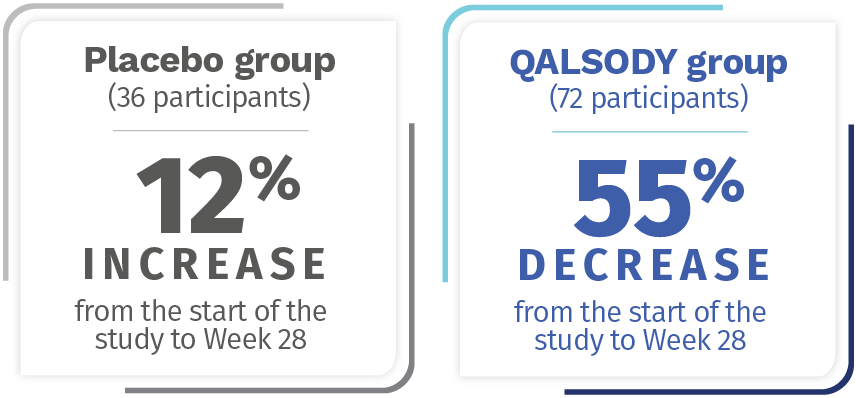
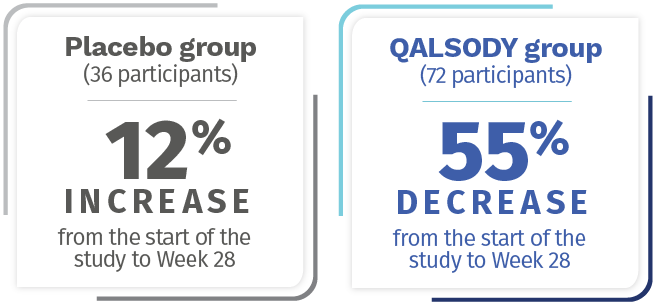
After 28 weeks, NfL levels in the blood decreased an average of 55% (Geometric mean ratio to baseline) from the start of the study in the QALSODY-treated participants and increased by 12% (Geometric mean ratio to baseline) from the start of the study in the placebo group. The Difference in geometric mean ratios between the QALSODY-treated participants and the placebo group was 60%.
NfL levels in the blood declined for approximately 113 days, then the reductions leveled off.
Difference in geometric mean ratios:
60% (95% CI): (0.33, 0.49)
Continued approval for QALSODY may depend on results of additional studies to confirm that there is a clinical benefit.
STAY INFORMED
Sign up to receive product information and
helpful resources.

